BECOME A MEMBER
Why Join NCODA?
NCODA is a grassroots, not-for-profit organization founded to strengthen oncology organizations with medically-integrated services. Our role is to be patient-centered community builders who connect medical teams with the vital information and resources they need. Membership with NCODA is complimentary.
Join a Growing Network of Cancer Care Professionals
We’re building a network of cancer care professionals whose focus is to innovate the continuity of cancer care so every patient receives the maximum benefit from their cancer treatment. As an NCODA member, you’ll have opportunities to collaborate—both in-person and virtually—with leading experts in oncology.
Access NCODA’s Complimentary Tools
Our members have access to a vast library of resources, including our PQIs, OCE and IVE sheets, publications including Oncolytics Today, and complimentary continuing education opportunities. Through our initiatives and committees, we’re continually leading the charge towards more effective, patient-focused care.
Stay Up-to-Date on Emerging Trends
Our members’ research is at the cutting edge of cancer care. Together, we’re improving continuity of care, enhancing quality systems, and improving patient outcomes.

Learn From Successful Medically-Integrated Practices
Our member practices have the opportunity to learn from other practices that are already successfully dispensing. Through NCODA, dispensing practices can come together to develop standards and best practices for delivering oral pharmaceuticals to oncology patients.
Physicians
The collaborative relationship that exists within medically-integrated organizations helps patients and physicians manage treatments in real-time, leading to improved care and patient adherence.
Pharmacists
Pharmacists are at the forefront of some of the most exciting trends in continuity of care and enhancing quality systems. NCODA provides the tools, resources, and connections necessary to help pharmacists thrive in medically-integrated practices.
Nurses
Nurses are vital to promoting the importance of the medically-integrated team and advocating for the improvement of managing patients receiving oral oncolytics.
Pharmacy Techs
By empowering pharmacy technicians through free continuing education and oncolytics resources, we can create better continuity of care for patients being treated with oral oncolytics.
Students
NCODA’s Professional Student Organization empowers the future generation of oncology pharmacy professionals. We support students through education, mentorship, meeting scholarships, professional development and post-graduate training.
Medically Integrated Staff
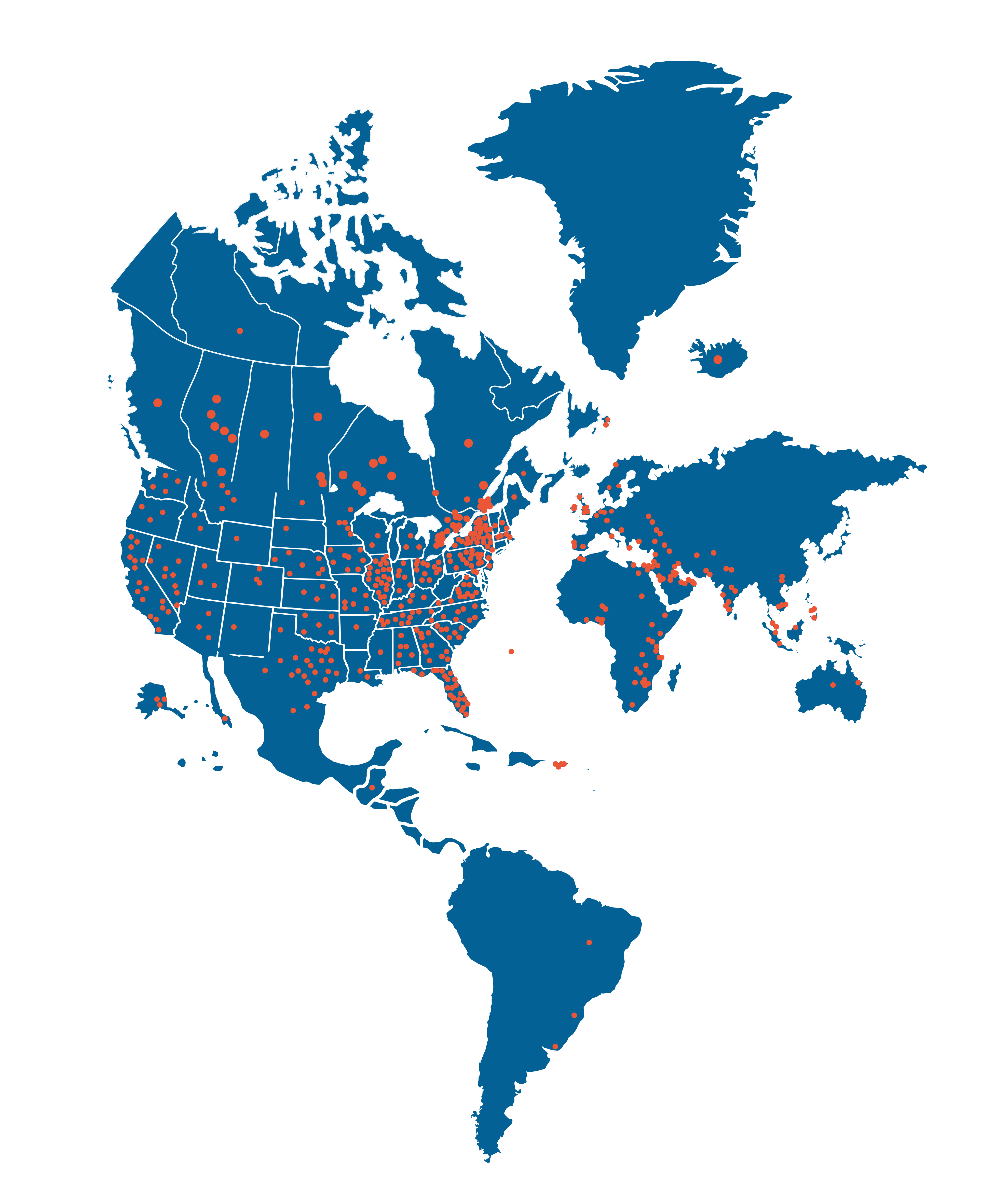
With membership in all 50 states and seven countries, NCODA is creating a global network of compassionate, patient-centered professionals.
NCODA
GLOBAL MEMBERSHIP
RESOURCES
Cost Avoidance Waste Tracker
PQI in Action
OCE Sheets
IVE Sheets
Treatment Support Kits
PQI Podcast
Members
Member Resources
OPTA
Committees
Member Login
Events
Webinars
Spring Forum
Oncology Institute
PSO Annual Meeting
Fall Summit
315-655-4640
P.O. Box 468
Cazenovia, NY 13035





